From buffer and media preparation, cell culture operations, purification operations up to final formulation, filtration and transfer, the sterile disconnection is a key element during manufacturing processes.
由医疗质量检测技术展知悉,从缓冲液和培养基的准备、细胞培养操作、纯化操作到最终配方、过滤和转移,无菌断开是制造过程中的关键要素。
It can seal either dry, wet or liquid-filled tubing in non-classified and classified environments while maintaining product sterility once the assemblies are disconnected.
封管机能够在非洁净和洁净环境中密封干燥、湿润或充满液体的管材,同时在组件断开连接后保持产品的无菌性。
2. Operating Principles工作原理
The TPE tubing to be disconnected is inserted into the Biosealer® TC. External clamps have to be added and closed according to the displayed instructions. The TPE tubing is first pressed, then heated up automatically to the defined temperature. When the heating phase is completed, the device cools down the sealing before its release.
将要断开连接的TPE管材插入Biosealer® TC 中,需要根据显示的指示添加并关闭外部夹具。TPE管材首先被压紧,然后自动加热到设定的温度。加热阶段完成后,设备在释放前先冷却密封部分。
List of Validated TPE Tubing Materials and Sizes Which Can Be Sealed on Biosealer® TC
可在Biosealer® TC 密封的经过验证的TPE管材材料和尺寸列表
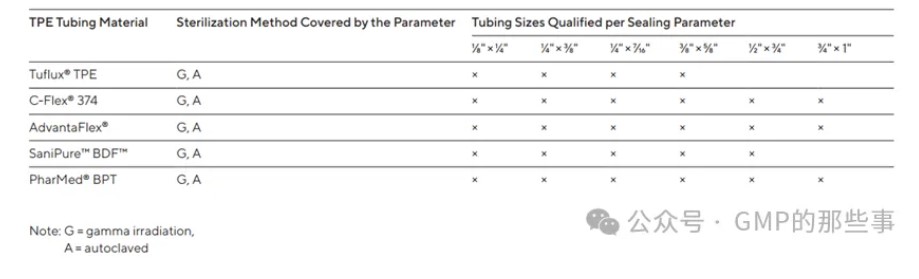
图片来源:GMP的那些事
3. Validation Tests验证测试
3.1 Validation Tests Overview验证测试概述
The following table summarizes all tests performed on each validated sealing parameters (see 2 for list of validated parameters):
下表总结了对每个已验证的密封参数(见章节2已验证参数列表)进行的所有测试。
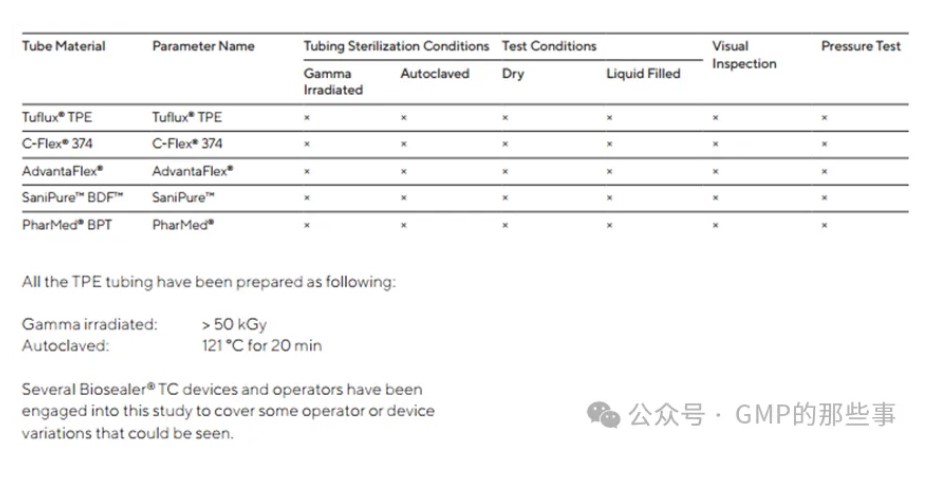
图片来源:GMP的那些事
-
3.2 Visual Inspection目视检查
3.2.1 Test Purpose测试目的
Several aspects are considered for visual inspection testing such as shape, surface or enclosures to secure the highest quality of the sealing. 目视检查会考虑多个方面,如形状、表面或封闭情况,以确保密封的最高质 量。
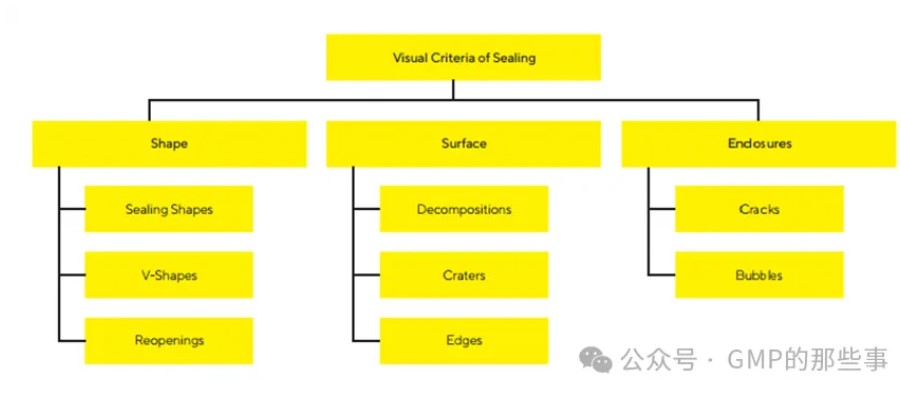
图片来源:GMP的那些事
Test Method测试方法
It consists of sealing TPE tubing with the parameter corresponding to a certain tubing material and dimension. The tubing is always sterilized according to the conditions specified in section 3.1. The resulting sealing is then visually inspected by trained operators to assess whether it has
passed or failed the visual inspection test.
它包括使用与特定管材材料和尺寸相对应的参数来密封TPE 管材。管材始终根据第3.1节指定的条件进行灭菌。然后,由训练有素的操作员对最终形成的密封进行目视检查,以评估它是否通过了目视检查测试。
-
3.3 Pressure Test压力测试
The pressure resistance of a sealing is a critical factor to be measured to avoid the sealing reopening, that would lead to a possible leakage and consequent contamination. 密封的耐压性是测量的关键因素,以避免密封重新打开,这可能导致泄漏和随之而来的污染。
3.3.1 Test Purpose测试目的
To submit a sealing to a certain pressure, mimicking the process conditions the sealing might be exposed to, ensuring the robustness of the sealing made with the defined parameters。
为了使密封件承受一定的压力,模拟密封件可能暴露的工艺条件,以确保按照规定参数制造的密封件的坚固性。
3.3.2 Test Method测试方法
To represent the worst-case scenario, gamma irradiated tubing was only used as well as dry conditions. A sealing is first made with Biosealer® TC and stored for five minutes. Then the sealing is cut into two sealed sections of 15 cm that are connected to pressurized air controlled by a manometer.
为了代表最坏情况,仅使用了伽玛辐照过的管材以及干燥条件。首先使用 Biosealer® TC进行密封,并存放五分钟。然后,将密封部分切割成两个15厘米长的密封段,这些段连接到由压力表控制的加压空气。

图片来源:GMP的那些事
The two sealing samples are submitted to a pressure of one bar for 60 seconds. The sealed tubing sections are then visually controlled to have no reopening of the sealing when reaching a critical limit.
将两个密封样品置于1bar压力下60秒。然后目视控制密封油管段,使其在达到临界极限时不会重新打开密封。
想要了解更多请前往医疗质量检测技术展
文章来源:GMP的那些事
若涉及侵权,请立刻联系删除
关键字:









